Because Time Matters
Qvella’s FAST Technology (Field Activated Sample Treatment) facilitates automation and acceleration of different stages of microbiology workflow to generate actionable information for bloodstream infections in minutes. FAST Technology includes preferential lysis of sample cells, rapid isolation and concentration of specific target cell types including pathogenic cells, extraction-free, in-line e-Lysis™ producing a clean, NAAT-ready lysate.
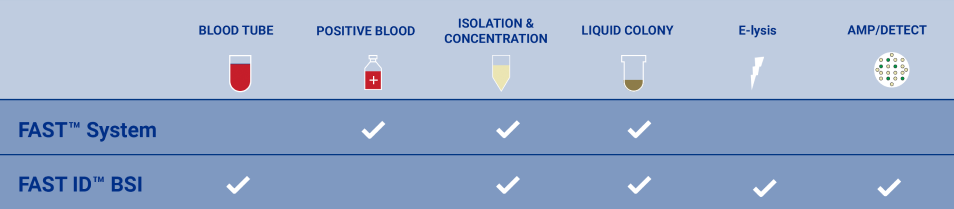
FAST-ID BSI System

Designed to detect over 90% of pathogens causing Sepsis in less than one hour directly from blood
- Surviving Sepsis Campaign (SSC)10 and UK National Institute for Health and Care Excellence (NICE)11 guidelines call for antimicrobial therapy within 1 hour of diagnosis of sepsis
- Every hour of delay in appropriate initial antibiotic therapy increases mortality by 7.6% associated with severe sepsis5
- Designed for detection of mono- or poly-microbial bloodstream infections in less than an hour
- Results reported as 16 individual test results at either the family, genus or species level
*FAST System and FAST PBC Prep Cartridge are listed as Class I IVD devices with the US FDA. These devices have not been validated for any downstream diagnostic applications and are only indicated for positive blood culture specimens containing microbial cells.
*FAST-ID™ is under development and not approved for sale. The performance characteristics have not been established.
1Sepsis Alliance (2016, November) Sepsis 2016 fact sheet Retrieved from https://www.sepsis.org/downloads/2016_sepsis_facts_media.pdf
2Dugani, S., Laxminarayan, R., & Kissoon, N. (2017). The quadruple burden of sepsis. Canadian Medical Association Journal, 189 (36), E1128-E1129.
3Rudd K. et al. (2020). Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: analysis for the Global Burden of Disease Study, Lancet, Vol.395, 200-210.
4National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013 (MAY 2016) HCUP Statistical Brief #204 Retrieved from https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf
5Kumar et al. (2015). Empiric Antimicrobial Therapy in Severe Sepsis and Septic Shock: Optimizing Pathogen Clearance. Curr Infect Dis Rep.,17(7), 493.
6Global Sepsis Alliance (2015, September) Retrieved from http://www.wfpiccs.org/wp-content/uploads/2015/09/2015_WSD_FactSheet_long_English.pdf
7European Commission: CORDIS: Projects and Results: Next generation sepsis diagnosis. (2016) Retrieved from Cordis.europa.eu. http://cordis.europa.eu/project/rcn/199396_en.html
8National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013 #204. (2016, May) Retrieved from Hcup-us.ahrq.gov. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp
9Lueangarun, S. & Leelarasamee, A. (2012). Impact of Inappropriate Empiric Antimicrobial Therapy on Mortality of Septic Patients with Bacteremia: A Retrospective Study. Interdisciplinary Perspectives on Infectious Diseases, Volume 2012, Article ID 765205, 13.
10Andrew Rhodes et al. (2017). Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock, Crit Care Med, 45(3), 486-552.
11National Institute for Health and Care Excellence Nice.org.uk (2017, September) Retrieved from https://www.nice.org.uk/guidance/qs161/chapter/Quality-statements
12McDonald et.al. (2005).Risk Factors for Ineffective Therapy in Patients with Bloodstream Infection, Arch Intern Med. 2005; 165308-313