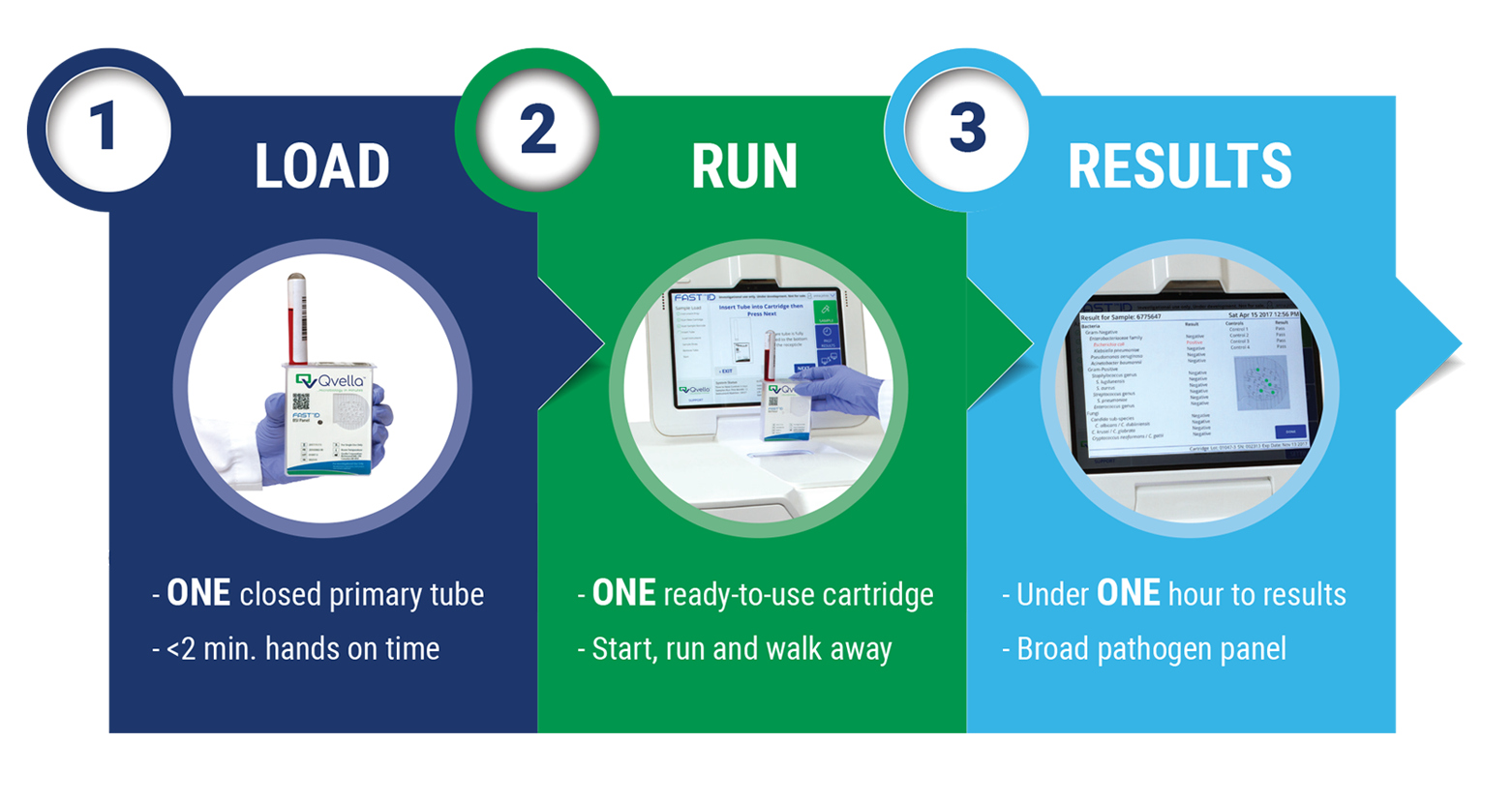

How It Works
Processing takes place in a matter of minutes
Pathogen Cell Isolation and Concentration
- Sealed vaccutainer whole blood sample
- Blood cells selectively lysed and debris removed
- Pathogen cells isolated and concentrated from whole blood sample
In-line, Automated Pathogen Cell Lysis, Amplification and Detection
- In-line e-lysis™ preserves nucleic acids and denatures inhibitors
- PCR-ready lysate is amplified for rRNA targets optimizing sensitivity
- 20 well array includes control and reaction wells
Results Available in Less than 60 Minutes
- Detects over 90% of pathogens causing bloodstream infections
- Bi-directional LIS interface
- Optional local printer for immediate availability of results
Comprehensive Pathogen Detection and Identification Directly From Whole Blood in Minutes
Qvella’s transformative FAST-TechnologyTM, in the FAST-ID BSI Panel is designed to deliver the unique ability to isolate and detect intact pathogens directly from a whole blood sample, providing identification of a wide range of pathogens causing BSI.
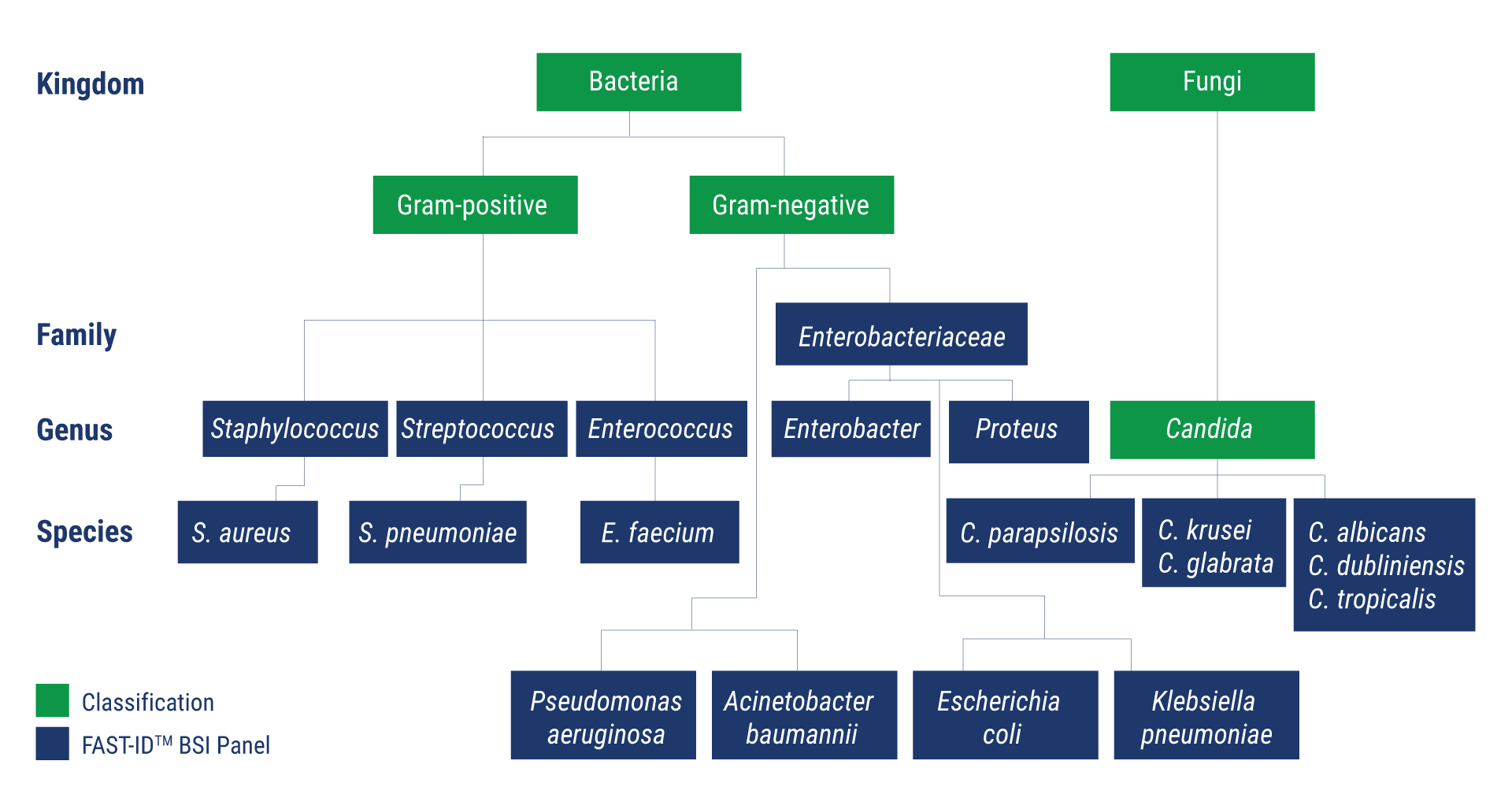
- rRNA target detection is designed for optimal sensitivity and specificity
- Designed to detect over 90% of sepsis causing bacteria and candida pathogens
- Identifies problematic species with challenging antibiotic resistance profiles
Actionable Results that can Meaningfully Impact Treatment Decisions
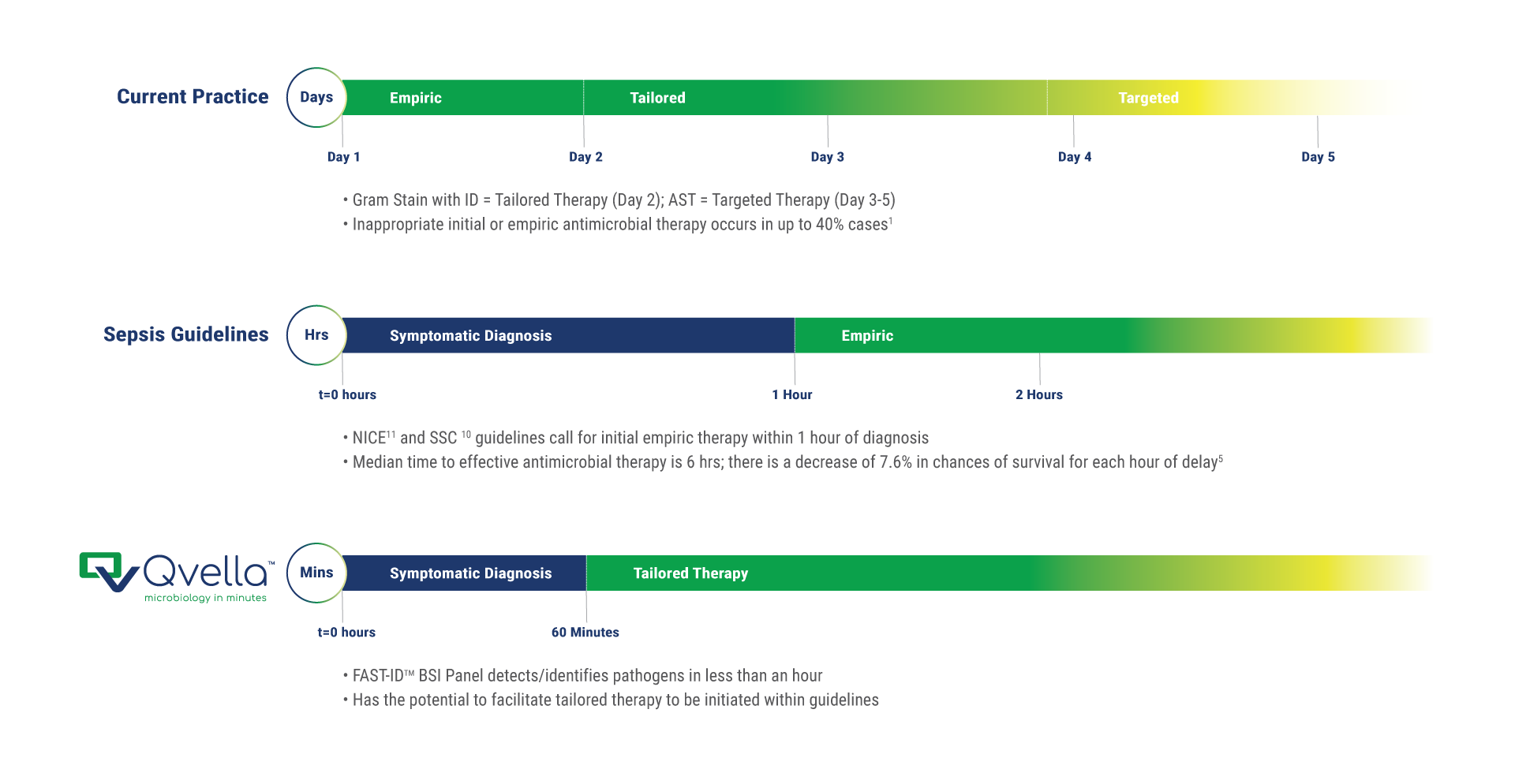
The critical difference for septic patients
- Earlier tailored antimicrobial therapy saves lives1
- SSC1 and NICE2 recommend initiation of antimicrobial therapy within one hour of diagnosis
- The FAST-IDTM BSI Panel is designed to provide results in minutes vs. hours
FAST Answers for Initial Therapy Decisions and Antimicrobial Stewardship
- Identifying infecting pathogens in BSI takes up to 5 days plus an additional 1+ day to AST
- This renders the earliest time for data-driven targeted (appropriate or optimal in the literature) antimicrobial therapy to 3 to 6 days
- The FAST-Technology™ BSI Panel is designed with the aim to deliver critical results to the clinical and stewardship teams to assist in defining the most appropriate antimicrobial therapies at the time of diagnosis.
*FAST-ID™ is under development and not approved for sale. The performance characteristics have not been established.